 |  |
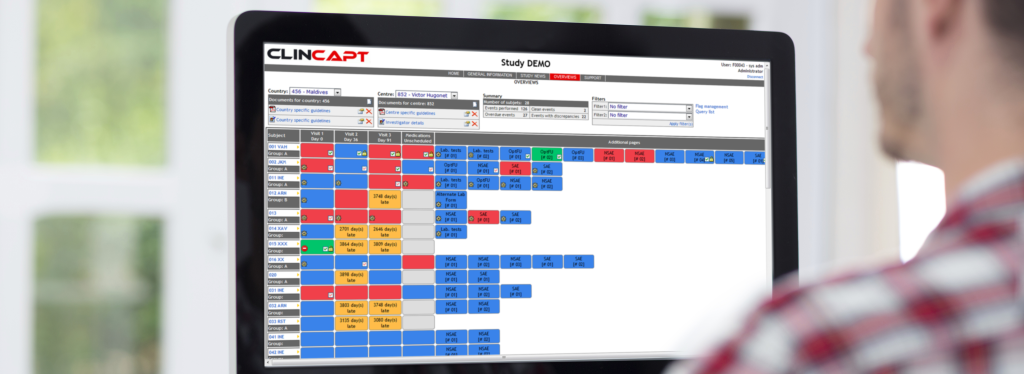
WEB PORTAL
A vital link between all study participants, the Internet portal gives controlled and secure access to:
- CRFs and queries, at their timely stage of completion, in PDF format for easy consultation
- Study information
- Site management
- Status reports, including data management, queries, adverse events, patient status, etc.
For EDC, the portal is of an even more critical nature, as it provides the communication interface for the investigators to enter and correct data as well as manage their patients, and for the monitors to closely follow and interact with their sites.
A number of routing processes can be implemented according to the specifications of each project allowing very effective distribution of information:
- Identification of specific occurrences in the CRF at a very early stage
- Notification to the appropriate departments
- Dedicated copy of the information to other involved bodies
- Sharing of functional processes between distant organizations
- Approval processes between different bodies

Vital link between
study participants
Critical
for EDC

Effective distribution
of information
| ©2016 i-clinics Ltd. All rights reserved. |